Recently, Scientists have accomplished what formerly was conserved for miracle workers: they have provided blind people the ability to see much better. In 2017, the vision field saw a massive advancement with the approval Luxturna, the initial genetics therapy to fix vision loss in specific patients with childhood years onset blindness.
And simply recently, researchers reported that a retinal implant enabled a 69-year-old woman with macular degeneration to more than double the number of letters she might determine on a vision chart.
” It’s early information however extremely promising, consisting of one patient with excellent vision gains, for an illness where we don’t have any type of treatment alternatives,” claims Thomas Albini of the University of Miami’s Bascom Palmer Eye Institute who was not involved in the research study.
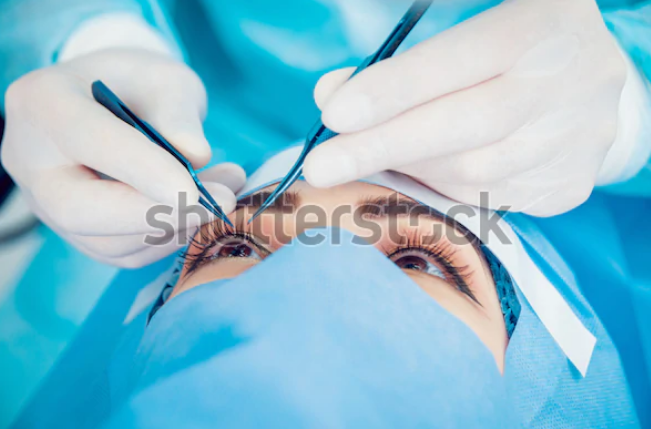
The implant, offered to five patients with dry age-related macular degeneration (AMD), is a single sheet of retinal pigment epithelial (RPE) cells originated from human embryonic stem cells. Various other groups across the globe are developing their very own type of RPE implants, and this type of approach is simply among a plethora of modalities being evaluated to either decrease or reverse various forms of blindness.
Up until now, the regenerative medicine field has actually identified exactly how to generate RPE cells, but various other cell types have proved more difficult “Getting photoreceptors or various other neurons inside the retina achieving which has been a golden target. No one has been successful in generating these cells,” says Jean Bennett, a professor of ophthalmology at the University of Pennsylvania.
Scientists have nonetheless developed creative technologies for sight restoration to deal with a variety of blindness conditions, some of which are enumerated below:
Cell implants
In the most recent advancement on dry AMD, published in Science Translational Medicine, a team of scientists and also engineers at the University of Southern California (USC) in Los Angeles initially evolved human embryonic stem cells (ESCs) into RPE cells – those that develop a monolayer that supports the health and wellness of the overlapping photoreceptor-harbouring retina. When spread out on an itsy-bitsy polymer platform (simply 6 mm by 3 mm), the cells assembled into a polarized mono-layer, just like that found in the human eye.
“What we have is a replica of the cells that are missing, because of degeneration, in patients with advanced dry AMD,” says Amir Kashani, a clinical scientist who performed the surgeries.
To insert the cells, Kashani made a one-millimetre incision in the retina, and with the implant folded in fifty percent “like a taco,” he moved it into the eye and unfolded it underneath the retina– which holds the patch in place – on top of the damaged area.
Three patients saw enhancements within three months of receiving the implant, including one patient who could check out additional letters on a vision chart. The other two patients had improvement in fixation, the capability to concentrate one’s gaze on an object.
Post-operative photographs of one subject 180 days (E) and another subject 120 days (F) after implantation. The bio engineered implant (black dotted line) is located near the area where vision cells have actually died (white dotted line).
The team is currently registering an additional 15 patients onto this trial and is planning a larger, phase 3 trial, additionally in dry AMD patients. “The objective is to try to fix much less severe disease so there is even more of a possibility for visual improvement,” says Kashani.
One more recent stem cell-based tissue approach from scientists in the United Kingdom resulted in vision improvement in two older patients with the wet form of AMD. In these cases, blood vessels in the eye grow abnormally and can lead to bleeding, the destruction of RPE cells, and eventual blindness. The developers also used a coated, synthetic membrane on which they assembled RPE cells differentiated from human ESCs and surgically delivered the eye spot. Two patients, a woman in her 60s and a man in his 80s, obtained the ability to read an additional 29 and 21 letters, respectively, on an analysis chart through a year. Each patient additionally had astonishing improvements in reading speed, from 1.7 to 82.8 and from 0 to 47.8 words per minute, respectively.
The initial presentation that human ESCs differentiated into RPE cells are safe was in 2012, when Steven Schwartz of the University of California, Los Angeles, and his colleagues effectively infused ESC -derived RPE cells into two legally blind patients, one with dry AMD and another with Stargardt’s macular dystrophy, one more degenerative eye disorder. In a 2014 follow up report of 18 patients, the team along with the manufacturer of the cells, Astellas Pharma, showed that 10 of the patients had some vision improvement. Schwartz and his colleagues are currently planning to register a bigger trial to evaluate if injecting a higher number of human ESC – derived RPE cells than used in the initial trial will certainly be efficacious.
One concern with using human ESCs is a prospective immune reaction, and subsequent rejection of the cells as foreign. To establish customized cell therapies originated from patients’ own cells, a group in Japan led by Masayo Takahashi at the RIKEN Centre for Developmental Biology have relied on reprogramming cells from skin fibroblasts into induced pluripotent stem (IPS) cells and then right into RPE cells. Her team recently revealed that a wet AMD patient who had a sheet of such RPE cells implanted into the eye did not have additional vision atrophy throughout a year.
There are likewise researchers working on a much more scalable approach, using donated, immune-matched IPS cells to treat several patients. A bank of IPS cells from a variety of donors would certainly be much more feasible, affordable + friendly, and also hassle-free than making IPS cells from each patient’s own cells.
Gene therapy and devices
To fix specific genetic defects associated with blindness disorders researchers have relied on gene therapy—and have achieved striking results. Luxturna, the gene therapy that FDA approved in 2015, fixes a mutation discovered in Leber congenital amaurosis (LCA) – a rare acquired form of childhood – and various other types of genetic retinal dystrophies harbouring a mutation in the RPE65 gene. The protein inscribed by the gene is an enzyme required for retinal cells to produce visual pigments. These inherited disorders generally start with night blindness and can proceed to a degeneration of peripheral vision and then total vision loss in childhood years.
One goal is to confer light level of sensitivity to retinal cells that usually do not have the capability to sense light.
An additional genetics therapy, still in the works, produces a healthy protein that obstructs the activity of vascular endothelial growth factor (VEGF) and has currently been shown to be safe and effective in an early-stage wet AMD clinical test.
A twist on gene therapy is an optogenetic approach in which a vector encoding a channel rhodopsin from environment-friendly algae is provided to retinal ganglion cells. The company Allergan is currently evaluating the strategy, developed by Zhuo-Hua Pan, a vision researcher at Wayne State University, in a beginning trial for patients with retinitis pigmentosa, in which patients lose light-sensing retinal cells. The objective, according to Pan, is to confer light sensitivity to retinal cells that usually don’t have the capability to sense light. While some genetics treatments, such as the one approved for LCA, target specific mutated genetics, optogenetic therapy might be utilized to deal with any type of blindness due to a loss of photoreceptor cells, says Pan.
Unproven stem cell techniques
The genetics and cell therapies in development are still in the early stages, and it’s not yet clear from the small numbers of patients dealt with up until now exactly how extensively appropriate they might be to people with vision loss. As cautious scientific research on these technologies inch their method towards ever-larger trials, there are centres offering unapproved treatments to customers with blindness – in spite of there being no stem cell – based therapies authorized for severe eye diseases.
Just recently, three women, each with macular degeneration, received an unverified stem cell treatment in both eyes in a clinic in Florida. All three were permanently blinded. Albini says the clinic’s protocol was a warning they weren’t operating very carefully. “The treatment ought to be performed in just one eye,” he says, whereas the stem cell clinic in Florida was providing its therapy to both eyes, blinding the females bilaterally.
An additional warning: the business model. “It’s important for patients to comprehend that legitimate clinical tests do not charge patients for the therapy,” adds Schwartz.
Regardless of the negative Public Relations from rogue players, ophthalmology researchers are bullish on what new treatments and advancements the following decades will certainly bring. Says Bennett, “It’s an exciting time. The vision is actually flourishing”.